April 9, 2020
As a follow-up to our March 13th bulletin, we are sharing an update regarding the availability of Hydrocortisone tablets. The NADF team continues to support our community by working with the FDA and pharmaceuticals regarding the issues our adrenal insufficiency patients have been experiencing with their difficulty filling this life-preserving drug.
On April 2, 2020 the FDA posted a Hydrocortisone tablet shortage. Any updates can be accessed here: https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Hydrocortisone%20Tablets,%20USP&st=c&tab=tabs-1
This is the most recent update from the FDA, and as verified as best as possible by NADF given the rapid changes in the availability and daily updates coming from manufacturers. More information will be provided as we continue to investigate, but in the meantime, here is the most up-to-date information.
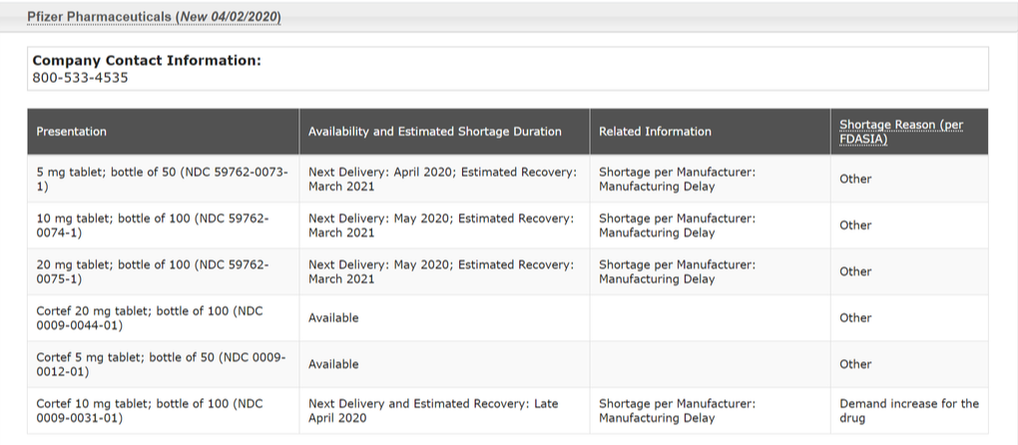
Pfizer is the predominant manufacturer of hydrocortisone, producing both the branded version known as Cortef and a generic version produced by a Pfizer subsidiary called Greenstone. In the table below (a screen shot of the FDA website), the top 3 rows indicate the status of Greenstone product, and the bottom 3 rows indicate the status of the branded (and more expensive and often restricted by insurance) branded Pfizer Cortef product.
As a follow-up to our March 13th bulletin, we are sharing an update regarding the availability of Hydrocortisone tablets. The NADF team continues to support our community by working with the FDA and pharmaceuticals regarding the issues our adrenal insufficiency patients have been experiencing with their difficulty filling this life-preserving drug.
On April 2, 2020 the FDA posted a Hydrocortisone tablet shortage. Any updates can be accessed here: https://www.accessdata.fda.gov/scripts/drugshortages/dsp_ActiveIngredientDetails.cfm?AI=Hydrocortisone%20Tablets,%20USP&st=c&tab=tabs-1
This is the most recent update from the FDA, and as verified as best as possible by NADF given the rapid changes in the availability and daily updates coming from manufacturers. More information will be provided as we continue to investigate, but in the meantime, here is the most up-to-date information.
Pfizer is the predominant manufacturer of hydrocortisone, producing both the branded version known as Cortef and a generic version produced by a Pfizer subsidiary called Greenstone. In the table below (a screen shot of the FDA website), the top 3 rows indicate the status of Greenstone product, and the bottom 3 rows indicate the status of the branded (and more expensive and often restricted by insurance) branded Pfizer Cortef product.
Per the FDA, Vensun product is available; Strides Pharma, who acquired Vensun, is still manufacturing. As indicated below.
Finally, while Amneal generic hydrocortisone may still be in circulation and some Rxs will be filled with Amneal product for now, they discontinued production some time ago.
Recommendations: Have a plan
With the COVID-19 pandemic and drug shortage it becomes even more challenging - and a plan is essential. It’s even more important for those of us with underlying conditions who are at higher risk. This outline attached will help you navigate through important resources to review and have handy as part of your plan.
You can be sure that NADF will continue to monitor the situation and update you on drug availability and other relevant COVID-19 advisories for our NADF members, families and friends. In the meantime, please stay healthy.
Recommendations: Have a plan
With the COVID-19 pandemic and drug shortage it becomes even more challenging - and a plan is essential. It’s even more important for those of us with underlying conditions who are at higher risk. This outline attached will help you navigate through important resources to review and have handy as part of your plan.
You can be sure that NADF will continue to monitor the situation and update you on drug availability and other relevant COVID-19 advisories for our NADF members, families and friends. In the meantime, please stay healthy.
Additional Information Regarding COVID-19
Quick Reference for Patients with Adrenal Insufficiency
Quick Reference for Patients with Adrenal Insufficiency
Protect yourself and be prepare for emergencies
Manage Your Medication
- Follow CDC guidelines for High Risk individuals. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
- Read the American Association of Clinical Endocrinologists (AACE) Coronavirus (COVID-19) position statement for people with Adrenal Insufficiency and Cushing’s Syndrome. https://www.aace.com/recent-news-and-updates/aace-position-statement-coronavirus-covid-19-and-people-adrenal
- Review NADF’s Disaster Preparedness Guidelines for Patients with Adrenal Insufficiency. Disaster Preparedness (pdf)
- Have an Emergency Kit Ready and refresh yourself on how to use your emergency injection. Injection Instructions
- Try to keep stress levels down
- Use the NADF Inspire forum to share and connect https://www.inspire.com/groups/national-adrenal-diseases-foundation/
- Follow the CDC Link to Managing Anxiety & Stress https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/managing-stress-anxiety.html
Manage Your Medication
- Try to have a 3-month supply of medication on hand
- Use Stress dosing guidelines Stress Dosing when you need it (pdf)
- Work with your doctor and pharmacy to find other options in the event of continued intermittent hydrocortisone drug shortages
- Try other brands: For example, if Greenstone hydrocortisone is not available, try Vensun or Pfizer if possible.
- Try other doses: If can’t get 5 mg, get 10 and cut the pills in half.
- Try other pharmacies: sometimes others will have it in their inventory.
- Contact a Compounding Pharmacy.
- Talk with your doctor about substituting with the equivalent dose with another Corticosteroid steroid.
- Follow CDC Updates https://www.cdc.gov/coronavirus/2019-ncov/index.html
- Follow NADF: www.nadf.us
NADF Website |
NADF Facebook Page |
NADF Twitter |
NADF |
NADF Virtual Support Forum |